Laboratory Information Management Systems (LIMS) have become a cornerstone in clinical labs, ensuring that operations run smoothly and with fewer errors. Whether it’s tracking samples, managing data, or generating reports, LIMS offers a centralized solution that saves time and reduces human error.
This article dives into how Laboratory Information Management System software enhances accuracy and streamlines processes in clinical laboratories. From its core features to the implementation steps, you’ll discover why more labs are turning to LIMS for better performance.
Key Features of LIMS Enhancing Accuracy
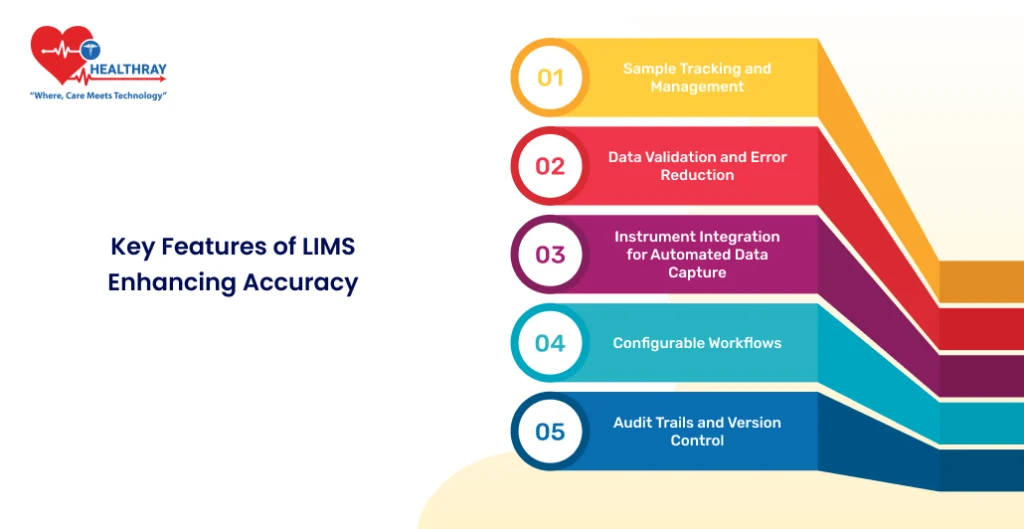
LIMS software offers a suite of features designed to boost accuracy in clinical labs. By reducing manual interventions and standardizing processes, it ensures consistent and error-free operations. Here’s how:
Sample Tracking and Management
One of the best LIMS software for clinical research to track samples through their lifecycle. LIMS provides real-time sample monitoring, reducing the chance of misplacement or mislabeling. Each sample is assigned a unique identifier, ensuring seamless traceability from collection to analysis.
Data Validation and Error Reduction
Human errors, such as transcription mistakes, are minimized with LIMS. It validates data entries by setting predefined rules and parameters, flagging any inconsistencies. This ensures that results meet quality standards before being finalized.
Instrument Integration for Automated Data Capture
LIMS connects directly with laboratory instruments, automating the process of transferring data. This eliminates the need for manual data entry, further reducing errors and speeding up workflows. For instance, test results from a hematology analyzer are directly imported into the system without intermediate steps.
Configurable Workflows
Clinical labs often have unique processes based on their specialties. LIMS software allows for workflow customization, ensuring that every procedure is adhered to without deviation. This consistency directly impacts the accuracy of results.
Audit Trails and Version Control
LIMS maintains detailed audit trails, tracking every change made within the system. This not only ensures accountability but also provides a clear record of data modifications. In case of any discrepancies, labs can review previous versions of records to identify and correct errors.
With these features, LIMS acts as a safeguard against inaccuracies that can compromise patient outcomes or compliance with regulations.
Enhancing Efficiency Through LIMS
Efficiency is critical in clinical laboratories, where delays can impact both operational costs and patient care. LIMS revolutionizes lab workflows by automating routine tasks, simplifying complex procedures, and optimizing resource utilization. Here’s how it helps:
Automation of Routine Tasks
Many repetitive processes, such as sample sorting, test scheduling, and report generation, are automated through LIMS. This reduces the time spent on manual tasks and allows lab personnel to focus on more critical activities. For instance, a LIMS system can automatically assign tests to appropriate instruments based on pre-set rules.
Streamlined Workflows
Clinical labs handle a variety of samples and tests daily. LIMS brings order by standardizing workflows and eliminating bottlenecks. From the moment a sample is registered to the final report delivery, every step is clearly defined and executed efficiently.
Real-Time Data Access and Collaboration
LIMS centralizes all lab data, making it accessible in real-time to authorized personnel. This fosters better collaboration between departments and ensures that lab managers have an up-to-date view of operations. For example, technicians can immediately flag abnormal test results to clinicians without delays.
Efficient Resource Management
Managing reagents, instruments, and staff schedules is simpler with LIMS. The system tracks inventory levels and instrument availability, ensuring that labs are always equipped to meet their workload. Alerts for low stock or overdue maintenance prevent interruptions in service.
Reduced Turnaround Times
Faster processes mean quicker delivery of test results, which is crucial in clinical settings. LIMS significantly reduces the time between sample collection and reporting by automating data transfers and minimizing human intervention.
By addressing inefficiencies, LIMS not only saves time but also reduces operational costs, enabling labs to handle higher volumes without compromising quality.
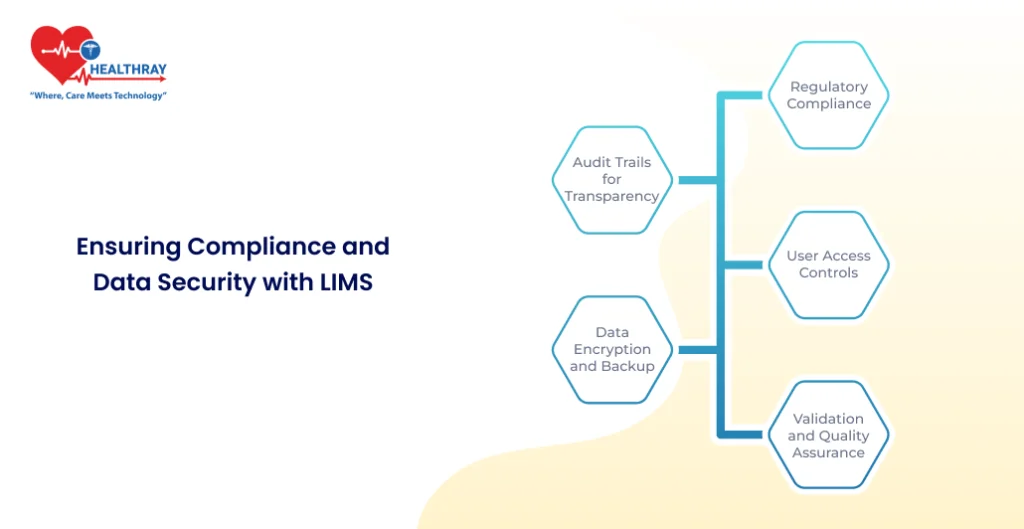
Ensuring Compliance and Data Security with LIMS
Clinical laboratories operate in a highly regulated environment, where compliance with laws and safeguarding patient data are top priorities. LIMS software is designed to help labs meet these requirements while maintaining data integrity and security.
Regulatory Compliance
LIMS supports compliance with industry standards such as CLIA (Clinical Laboratory Improvement Amendments), HIPAA (Health Insurance Portability and Accountability Act), and ISO 15189. It ensures all processes, from sample handling to result reporting, align with regulatory requirements by automating documentation and maintaining detailed records.
Audit Trails for Transparency
Every action taken within the LIMS system is logged with time stamps and user information. This creates an unalterable audit trail that labs can use to demonstrate compliance during inspections or audits. Whether it’s a change to a patient record or an update to a test protocol, everything is traceable.
User Access Controls
Data breaches can have severe consequences in clinical labs. LIMS minimizes risks by restricting access to sensitive information. Role-based permissions ensure that users can only view or modify data relevant to their responsibilities, reducing the likelihood of unauthorized access.
Data Encryption and Backup
Patient data stored in a LIMS system is encrypted to prevent unauthorized access. Additionally, automated backups ensure that critical information is not lost due to system failures or other unforeseen events. These measures enhance overall data security.
Validation and Quality Assurance
LIMS includes built-in quality checks to validate data at every stage. This prevents errors from propagating and ensures the integrity of final results. Regular system updates and validations also help maintain compliance with evolving regulatory standards.
By integrating compliance and security into daily operations, LIMS not only protects labs from legal and financial risks but also builds trust with patients and stakeholders.
Cost-Benefit Analysis of Implementing LIMS
Investing in a Laboratory Information Management System (LIMS) might seem like a significant expense at first, but the long-term savings and operational benefits often outweigh the initial costs. Here’s a breakdown of the financial and practical aspects to consider:
Initial Investment
The upfront costs for LIMS include software licenses, hardware, implementation, and training. While this might seem substantial, many LIMS providers offer scalable solutions, allowing labs to choose a system that fits their size and budget.
Operational Cost Savings
Automation of manual tasks significantly reduces the need for labor-intensive processes, which can save on staffing costs. Additionally, the best LIMS software for clinical research minimizes errors that lead to repeat tests or wasted resources, providing direct savings in consumables and reagents.
Reduction in Errors and Associated Costs
Errors in clinical labs can be costly, both financially and in terms of reputation. By improving data accuracy and standardizing workflows, LIMS reduces these errors, leading to fewer corrective actions and associated costs.
Time Savings and Productivity Gains
Faster processing times translate to increased productivity. With LIMS handling routine operations, lab personnel can focus on higher-value tasks like analysis and innovation. This allows labs to manage larger volumes of work without additional staff.
Improved Regulatory Compliance
Non-compliance with healthcare regulations can result in heavy fines or operational shutdowns. LIMS helps avoid these penalties by ensuring adherence to regulatory requirements and maintaining thorough documentation for audits.
Long-Term ROI
Over time, the efficiency, accuracy, and compliance benefits of LIMS lead to a significant return on investment (ROI). Clinical labs often find that the system pays for itself through reduced operational costs and increased throughput.
Implementation Considerations for LIMS in Clinical Labs
Introducing a Laboratory Information Management System (LIMS) into a clinical lab can be transformative, but it requires careful planning to ensure a smooth transition. Here’s what labs should consider during implementation:
Assessment of Lab Needs
Before choosing a LIMS, labs should identify their specific requirements. Consider factors like the volume of samples processed, the type of tests conducted, and the need for integration with existing equipment. A clear understanding of these needs will guide the selection of a system that fits best.
Vendor Selection
Not all LIMS solutions are created equal. Labs should evaluate vendors based on their reputation, customer support, and ability to customize the software. Requesting demos and case studies can provide insights into how well the solution has performed for similar labs.
System Customization and Scalability
Clinical labs often have unique workflows. A good LIMS allows for customization to align with these processes. It should also be scalable to accommodate future growth, such as higher sample volumes or additional testing capabilities.
Data Migration and Integration
Transferring existing data into the new system is a critical step. This process must be planned to avoid data loss or inconsistencies. Additionally, integrating LIMS with lab instruments, databases, and other systems ensures seamless data flow across the lab.
Training and Change Management
Successful LIMS implementation depends on user adoption. Comprehensive training sessions should be conducted for all lab staff to familiarize them with the system. Clear communication about the benefits and changes to workflows can ease the transition.
Testing and Validation
Before going live, the LIMS should be thoroughly tested to ensure it meets the lab’s needs. Validation ensures that all workflows function as expected, data is accurate, and regulatory requirements are met.
Ongoing Support and Maintenance
Implementation doesn’t end at launch. Continuous support from the vendor is essential for troubleshooting and updates. Regular maintenance and upgrades keep the system running optimally and compliant with evolving regulations.
Pro Tip: Start small by rolling out the system in one department or for a single workflow. Once the team is comfortable, expand its use across the entire lab.
Proper planning and phased implementation can minimize disruptions and maximize the benefits of LIMS for clinical labs.
Case Studies and Success Stories
Real-world examples can demonstrate how implementing LIMS transforms clinical lab operations, improving accuracy, efficiency, and compliance. Here are a couple of success stories to highlight the tangible benefits of LIMS:
Mid-Sized Clinical Lab: Reducing Turnaround Times
- Challenge: A mid-sized clinical lab faced delays in processing patient samples due to manual data entry and inefficient sample tracking. This resulted in frequent errors and longer turnaround times, impacting patient satisfaction.
- Solution: The lab implemented a customized LIMS to automate data entry and integrate with its lab instruments. The system also provided real-time sample tracking and automated test result reporting.
- Outcome: Within six months, the lab reduced its average turnaround time by 40%. Sample misplacement incidents dropped to zero, and staff productivity increased significantly as manual tasks were eliminated.
Large Healthcare Network: Ensuring Compliance Across Multiple Locations
- Challenge: A large healthcare network with multiple labs struggled to maintain consistent compliance with regulatory standards across locations. Each lab used its own system, leading to inconsistent workflows and documentation.
- Solution: The network adopted a centralized LIMS solution that standardized processes and ensured adherence to compliance requirements like CLIA and HIPAA. The system provided a unified platform for all labs, with customizable workflows to meet local needs.
- Outcome: The centralized LIMS improved compliance reporting efficiency by 50%. Standardized workflows reduced errors across locations, and the unified platform allowed management to oversee operations in real-time.
Specialty Clinical Lab: Managing High Sample Volumes
- Challenge: A specialty clinical lab handling high volumes of genetic testing samples found its manual tracking methods could not keep up, leading to frequent bottlenecks and errors.
- Solution: The lab implemented a scalable LIMS designed for high-throughput environments. Features like automated sample labeling, instrument integration, and streamlined reporting processes were deployed.
- Outcome: The lab increased its sample processing capacity by 60%, meeting the demands of a growing client base without compromising accuracy. Cost savings from reduced errors and improved efficiency offset the initial investment within a year.
These case studies highlight how LIMS can address specific challenges in clinical labs and deliver measurable improvements in operations.
Conclusion
Implementing a Laboratory Information Management System (LIMS) can be a game-changer for clinical laboratories. From reducing errors and improving data accuracy to streamlining workflows and ensuring regulatory compliance, the benefits are substantial. Labs that adopt LIMS often experience faster turnaround times, lower operational costs, and enhanced productivity.As clinical labs face growing demands and tighter regulations, a robust Hospital Management System solution isn’t just an upgrade—it’s a necessity. By investing in the right system and planning for a smooth implementation, labs can position themselves for long-term success while delivering reliable results for patients.