Managing diagnostic processes efficiently is critical for modern laboratories. A Lab Diagnosis Management System (LDMS) helps labs streamline workflows, reduce errors, and ensure compliance with industry standards.
If you’re a Healthcare IT professional, you might be concerned about how to integrate such a system with existing infrastructure while maintaining data security. Laboratory managers often wonder how an LDMS can simplify daily operations and improve turnaround times. Meanwhile, hospital administrators are likely looking at cost-effectiveness and the overall return on investment.
This guide unpacks everything you need to know about implementing a Lab Diagnosis Management System—from assessing your lab’s needs to going live with a robust, reliable system. We’ll also tackle common challenges and offer actionable insights for a smoother implementation process.
Planning and Preparation
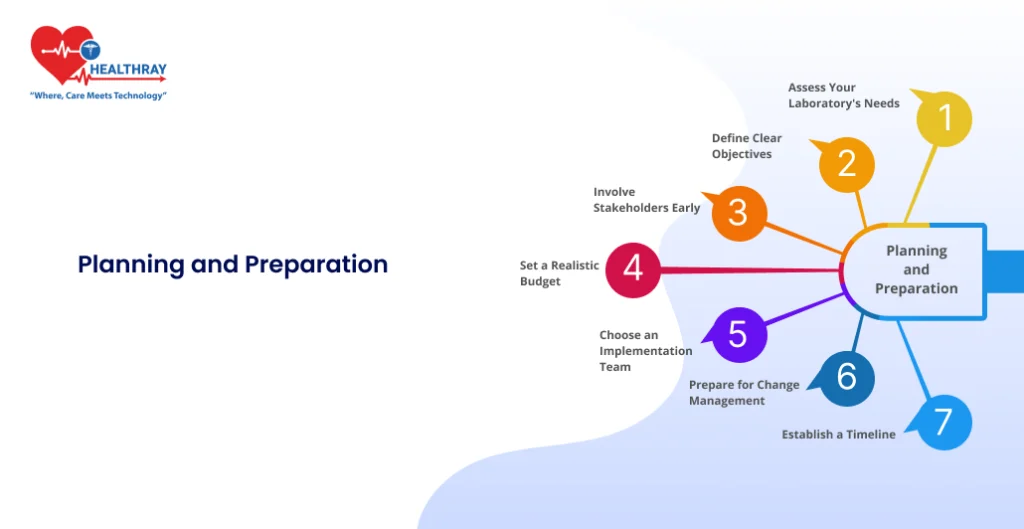
A strong foundation makes everything else smoother. Implementing a Lab Diagnosis Management System starts with meticulous planning. This phase sets the stage for a successful rollout by ensuring your objectives, resources, and expectations are aligned. Here’s how you can approach this step:
Assess Your Laboratory’s Needs
- Evaluate current workflows and identify inefficiencies.
- List specific features your lab requires (e.g., data visualization, automated reporting, compliance tools).
- Consider the volume of tests your lab processes daily to ensure the system can handle future scalability.
Define Clear Objectives
- Clarify what you aim to achieve. Is it faster turnaround times, improved compliance, or better data management?
- Align these objectives with stakeholders, including lab staff, IT teams, and hospital leadership.
Pro Tip: Document these goals. They will serve as benchmarks to measure the success of your implementation later.
Involve Stakeholders Early
- Include representatives from every department that will interact with the system: IT, lab staff, and administrative teams.
- Schedule regular meetings to gather feedback, address concerns, and maintain alignment.
Set a Realistic Budget
- Factor in costs for software, hardware (if needed), staff training, and ongoing maintenance.
- Plan for contingencies like unexpected equipment upgrades or extended training.
Choose an Implementation Team
- Designate a project lead to oversee the process and coordinate between teams.
- Include specialists familiar with the lab’s workflows and IT professionals for technical support.
Prepare for Change Management
- Communicate the benefits of the system to staff early. Resistance often stems from a lack of understanding.
- Develop a change management strategy to guide staff through the transition and address their concerns.
Establish a Timeline
- Create a realistic implementation schedule, breaking it into manageable phases: planning, configuration, testing, and go-live.
- Allow extra time for testing and training, as these are critical for success.
By following these steps, you can ensure a solid foundation for the successful implementation of your Lab Diagnosis Management System. A well-prepared team with clear goals and expectations minimizes disruptions and keeps the project on track.
Selecting the Right System
Choosing the right Lab Diagnosis Management System is a critical step in the implementation process. The system you select will directly impact efficiency, compliance, and staff satisfaction. Here’s how to ensure you make the best choice for your lab.
Understand Key Features
Identify the essential features your lab needs. While different systems offer varying capabilities, some key functionalities to look for include:
- Data Integration: Ability to connect with laboratory instruments and hospital information systems.
- Automated Reporting: Streamlined creation of test reports to reduce manual effort.
- Regulatory Compliance: Features that ensure alignment with industry standards like HIPAA, CLIA, or ISO 15189.
- Real-Time Data Access: Instant access to data for quick decision-making and operational efficiency.
Evaluate Vendors Thoroughly
All systems are not created equal. Before committing to a vendor, consider:
- Reputation and Experience: Look for vendors with proven track records in healthcare IT and lab management.
- Customer Support: Check for availability of 24/7 support, especially during the early stages of implementation.
- Customization Options: Ensure the system can be tailored to suit your lab’s unique needs.
Assess System Scalability
Think long-term. Your lab may grow or adopt new technologies, so ensure the system can scale with you. Key questions to ask:
- Can the system handle increased test volumes?
- Will it integrate with new equipment or software in the future?
- Is there an option to upgrade features without replacing the entire system?
Consider User-Friendliness
A sophisticated system is only as good as its usability. Ensure that:
- The interface is intuitive and easy to navigate.
- Training requirements for staff are manageable.
- Errors in data entry or reporting are minimized through automation or built-in checks.
Prioritize Data Security
With sensitive patient data at stake, security is paramount. Verify that the system offers:
- End-to-end encryption for data protection.
- Role-based access control to limit access to sensitive information.
- Regular software updates to address security vulnerabilities.
Compare Costs
Budget considerations will play a significant role in your decision. When comparing costs:
- Look beyond the upfront price to include maintenance, updates, and support.
- Weigh the potential savings from improved efficiency against the system’s total cost of ownership (TCO).
Request Proposals and Evaluate Offers
Ask shortlisted vendors for detailed proposals, including:
- Feature specifications.
- Implementation timelines.
- Post-sale support plans.
Implementation Steps
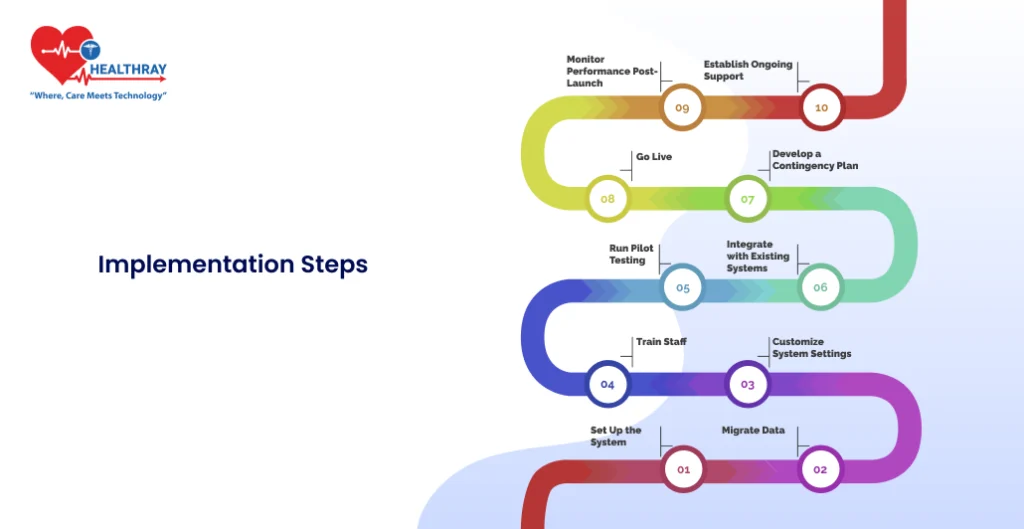
Once you’ve selected the right Lab Diagnosis Management System, the real work begins. The implementation phase is critical, as it determines how well the system integrates into your lab’s workflow. Here’s a step-by-step guide to ensure a smooth and efficient implementation process.
Set Up the System
- Software Installation: Collaborate with your vendor to install and configure the software on your servers or cloud platform.
- Hardware Integration: Connect laboratory instruments, computers, and other devices to the system. Ensure compatibility and test connections thoroughly.
Migrate Data
- Transfer existing patient records, test results, and inventory data into the new system.
- Cleanse data before migration to eliminate duplicate or outdated information.
Customize System Settings
- Configure workflows to align with your lab’s processes. For example:
- Sample collection and tracking.
- Automated alerts for critical results.
- Report generation formats.
- Set user roles and permissions to maintain security and streamline tasks.
Train Staff
- Conduct training sessions tailored to different user roles. For instance:
- IT teams should learn system maintenance and troubleshooting.
- Lab technicians need to know sample logging and test processing.
- Administrators must understand reporting and compliance features.
- Provide hands-on practice through a test environment to build confidence.
Run Pilot Testing
- Implement the system on a small scale to identify gaps or issues.
- Use pilot testing to:
- Validate system configurations.
- Gather feedback from staff on usability and performance.
Integrate with Existing Systems
- Connect the LDMS with other systems like:
- Hospital Information Management System (HIMS).
- Billing and inventory management software.
- Test integrations to ensure seamless data exchange and communication.
Develop a Contingency Plan
- Prepare for potential disruptions during implementation. This includes:
- Temporary backup processes for sample tracking and reporting.
- IT support to address unexpected issues.
- Have a rollback plan in case of critical failures during go-live.
Go Live
- Roll out the system across the lab. Ensure:
- Adequate IT and vendor support is available during the first week.
- Staff can quickly access troubleshooting resources.
- Monitor the transition closely to address any immediate concerns.
Monitor Performance Post-Launch
- Track key performance indicators (KPIs) such as:
- Turnaround time for test results.
- Error rates in reporting or data entry.
- User satisfaction based on staff feedback.
- Use these metrics to identify areas for further optimization.
Establish Ongoing Support
- Partner with the vendor for regular software updates and maintenance.
- Set up an internal support team to handle day-to-day issues.
Ensuring Compliance and Data Security
One of the most critical aspects of implementing a Lab Diagnosis Management System (LDMS) is ensuring it complies with regulatory standards and maintains robust data security. Labs deal with sensitive patient data, and any breach or non-compliance can lead to significant legal and financial consequences. Here’s how to address these priorities effectively.
Understand Relevant Regulations
- Familiarize yourself with local and international standards applicable to your lab. Common regulations include:
- HIPAA (Health Insurance Portability and Accountability Act): For labs handling patient data in the U.S.
- CLIA (Clinical Laboratory Improvement Amendments): For ensuring quality standards in laboratory testing.
- GDPR (General Data Protection Regulation): For labs operating in Europe, focusing on data privacy.
- ISO 15189: For quality and competence in medical laboratories.
Conduct a Compliance Audit
- Before implementing the system, assess your current operations against regulatory requirements.
- Identify gaps and develop a plan to address them, ensuring the new system aligns with these standards.
Establish Data Privacy Protocols
- Implement strict data access controls. Role-based permissions ensure that only authorized personnel can access sensitive information.
- Encrypt data both in transit and at rest to prevent unauthorized access during storage or transmission.
- Regularly update passwords and security settings to stay ahead of potential breaches.
Ensure System Reliability
- Conduct regular system performance checks to avoid downtimes that could disrupt operations and compliance reporting.
- Implement failover systems or backup servers to ensure uninterrupted access to critical data.
Train Staff on Compliance
- Educate staff about the importance of regulatory compliance and data security.
- Provide training on how to handle sensitive data, recognizing phishing attempts, and using the LDMS responsibly.
Conduct Security Assessments
- Perform vulnerability tests and penetration testing to identify and address weaknesses in the system.
- Partner with cybersecurity experts to audit your system’s security regularly.
Maintain an Incident Response Plan
- Develop a clear plan for managing potential breaches. This should include:
- Immediate actions to contain and assess the breach.
- Communication protocols for notifying affected parties and regulatory bodies.
- Steps to resolve vulnerabilities and prevent future incidents.
Document and Archive for Audits
- Keep detailed records of all lab activities, including:
- Test processes and results.
- Data access logs.
- Incident reports.
- Ensure the LDMS supports automatic documentation and archiving to simplify compliance audits.
Monitor for Updates in Regulations
- Stay informed about changes in industry regulations or standards. Regularly update your LDMS to meet new requirements.
- Engage with professional organizations or compliance bodies to stay ahead of regulatory trends.
Work Closely with Your Vendor
- Choose a vendor that prioritizes compliance and security in their system updates.
- Ensure they provide tools like audit trails, encryption options, and compliance checklists.
By proactively addressing compliance and data security, your lab can protect sensitive patient data, meet regulatory obligations, and maintain trust with stakeholders. This approach also minimizes risks, ensuring your operations run smoothly and securely.
Staff Training and Change Management

Implementing a Lab Diagnosis Management System (LDMS) involves more than just technical changes. Success hinges on your staff’s ability to adapt to new workflows and embrace the system. Proper training and thoughtful change management ensure a seamless transition. Here’s how to approach this phase effectively.
Identify Training Needs
- Assess the roles and responsibilities of each team member to tailor training sessions:
- Lab technicians: Focus on test entry, results tracking, and equipment integration.
- IT staff: Cover system troubleshooting, updates, and data management.
- Administrators: Train on compliance tools, reporting, and overall system monitoring.
Develop a Training Program
- Create a structured training plan that covers:
- System navigation and daily operations.
- New workflows enabled by the LDMS.
- Troubleshooting common issues.
- Use a mix of formats to cater to different learners:
- Hands-on practice in a test environment.
- Step-by-step guides and video tutorials.
- Interactive workshops for collaborative learning.
Appoint Superusers
- Identify team members who can serve as “superusers” or champions of the new system. These individuals:
- Receive advanced training.
- Act as go-to resources for their peers.
- Help troubleshoot minor issues during and after implementation.
Communicate the Benefits
- Address resistance by clearly explaining how the LDMS will improve daily tasks:
- Faster test processing.
- Fewer manual errors.
- Easier access to data and reports.
- Highlight success stories from other labs to build confidence in the system.
Run Pilot Sessions
- Before a full rollout, conduct pilot training sessions to:
- Identify areas where staff may need extra guidance.
- Gather feedback on the training materials.
- Fine-tune the program based on real-world usage.
Provide Continuous Support
- Offer ongoing training opportunities post-implementation, such as:
- Refresher courses for existing staff.
- Training modules for new hires.
- Keep support materials like FAQs, guides, and video tutorials easily accessible.
Foster a Culture of Change
- Encourage staff to embrace the new system by:
- Recognizing and rewarding those who adapt quickly.
- Creating an open feedback loop where staff can share challenges and suggestions.
Measure Training Effectiveness
- Evaluate the success of your training program through:
- Staff proficiency tests to assess their understanding.
- Surveys to gather feedback on the training process.
- Monitoring error rates and workflow efficiency post-training.
Anticipate and Address Resistance
- Common sources of resistance include fear of increased workload, lack of technical skills, and unfamiliarity with the system.
- Address these by:
- Offering extra support to hesitant staff.
- Emphasizing the long-term benefits of the system.
- Providing time for gradual adaptation.
Celebrate Milestones
- Recognize progress to keep morale high. For example:
- Acknowledge staff who complete training early.
- Celebrate successful pilot tests or the system’s go-live day.
By investing in comprehensive training and managing change thoughtfully, you equip your team with the tools and confidence they need to succeed. This ensures smoother adoption and maximizes the benefits of your new Lab Diagnosis Management System.
Testing and Validation
Testing and validation are crucial steps in the implementation of a Lab Diagnosis Management System (LDMS). These steps ensure the system works as intended and integrates seamlessly into your laboratory’s operations. A thorough testing process minimizes errors, identifies potential issues early, and builds confidence before the system goes live.
Develop a Testing Plan
- Define clear objectives for testing, such as:
- Verifying system functionality.
- Ensuring data accuracy during migration.
- Validating integrations with existing hardware and software.
- Create a detailed checklist covering all workflows, including:
- Sample entry and tracking.
- Test result generation.
- Reporting and data retrieval.
Conduct Unit Testing
- Test individual components of the system to ensure they function correctly on their own.
- Key areas to test include:
- User interfaces for ease of navigation.
- Instrument connections for accurate data transfer.
- Reporting modules for formatting and compliance.
Run Integration Testing
- Ensure the LDMS integrates seamlessly with existing systems such as:
- Hospital Information Systems (HIS).
- Billing and inventory management software.
- Test data exchange between systems to confirm accuracy and speed.
Test Data Migration
- Validate that historical data has been accurately migrated to the new system. This includes:
- Checking for data integrity (no missing or duplicated records).
- Ensuring all fields are properly mapped to their new locations.
Simulate Real-World Scenarios
- Conduct end-to-end testing by simulating actual lab processes, such as:
- Logging a sample.
- Running diagnostic tests.
- Generating and delivering a report.
- Observe system behavior under varying workloads to ensure stability.
Involve End Users
- Have lab technicians, administrators, and IT staff participate in testing. This helps:
- Identify usability issues.
- Gather feedback on system performance and workflow alignment.
- Build familiarity with the system before it goes live.
Pilot Testing
- Implement the system in a controlled environment, such as one department or a small-scale operation.
- Use this phase to:
- Measure system performance in real-world conditions.
- Address issues reported by pilot users.
- Fine-tune workflows and settings.
Monitor and Adjust
- Analyze the results of your tests to:
- Identify recurring issues.
- Adjust system configurations as needed.
- Document all changes to ensure they align with compliance standards.
Validate Against Regulatory Requirements
- Ensure the system meets all necessary compliance standards (e.g., HIPAA, CLIA, GDPR).
- Verify that audit trails, access controls, and reporting features are functional and aligned with regulatory needs.
Sign Off on Go-Live Readiness
- Once all tests are complete and issues resolved, conduct a final review to confirm the system is ready for deployment.
- Include sign-offs from key stakeholders, such as:
- IT leads for technical validation.
- Lab managers for workflow approval.
- Compliance officers for regulatory adherence.
By prioritizing testing and validation, you minimize risks and ensure your LDMS operates flawlessly in your laboratory’s environment. This step lays the groundwork for a successful go-live and long-term system reliability.
Go-Live and Post-Implementation Support

The go-live phase is the culmination of all your preparation, testing, and training efforts. It marks the official deployment of your Lab Diagnosis Management System (LDMS) in your lab’s operations. However, the work doesn’t stop there. Post-implementation support ensures the system continues to function optimally while addressing any challenges that arise.
Plan a Soft Launch
- Begin with a soft launch, where the system is implemented during a low-activity period. This allows your team to:
- Observe the system in real-world conditions.
- Identify and resolve minor issues without significant disruptions.
- Gradually increase usage to full capacity over a predetermined timeline.
Ensure On-Site Support
- Arrange for vendor representatives or IT specialists to be on-site during the go-live period.
- Provide immediate assistance for:
- Troubleshooting errors.
- Answering staff questions.
- Adjusting workflows if necessary.
Monitor System Performance
- Track key performance indicators (KPIs) to evaluate the system’s success. Examples include:
- Turnaround time for test results.
- System uptime and reliability.
- Staff satisfaction with the system.
- Use these metrics to identify areas needing improvement.
Collect Feedback
- Encourage staff to share their experiences during the initial weeks of implementation. Focus on:
- Ease of use.
- Workflow integration.
- Any recurring issues or bottlenecks.
- Use surveys or team meetings to facilitate open and honest feedback.
Address Initial Challenges
- Common issues during go-live may include:
- Data entry errors due to unfamiliarity with the system.
- Minor technical glitches.
- Workflow misalignments.
- Create a prioritized action plan to resolve these challenges swiftly.
Schedule Regular System Updates
- Coordinate with the vendor to implement software updates and patches that improve functionality and address security vulnerabilities.
- Ensure updates are tested in a controlled environment before applying them to the live system.
Provide Continuous Training
- Offer additional training sessions for staff to:
- Reinforce key workflows.
- Address questions arising from real-world usage.
- Acquaint new employees with the system.
- Keep training resources like guides and video tutorials up-to-date.
Establish Ongoing Support Channels
- Set up a dedicated help desk or support team to address system-related issues post-implementation.
- Maintain open communication with the vendor for advanced troubleshooting or system upgrades.
Document Lessons Learned
- After the system has been live for a few months, conduct a review to:
- Document what went well and what could have been improved.
- Create a roadmap for future enhancements or system expansions.
- Share findings with all stakeholders to align on next steps.
Celebrate the Achievement
- Recognize the efforts of everyone involved in the implementation process. This could include:
- Acknowledging staff contributions in meetings or newsletters.
- Hosting a small event to mark the successful go-live.
- Celebrating milestones boosts morale and fosters enthusiasm for future projects.
By carefully managing the go-live phase and maintaining robust post-implementation support, your lab can maximize the benefits of the LDMS. These steps ensure the system continues to operate smoothly, evolves with your lab’s needs, and delivers long-term value.
Measuring Success
After implementing a Lab Diagnosis Management System (LDMS), measuring its success is essential to understand its impact on your lab’s operations. Tracking performance helps identify improvements, justify the investment, and ensure the system meets your goals. Here’s how to evaluate its effectiveness:
Define Key Performance Indicators (KPIs)
- Establish measurable metrics to assess the system’s performance. Common KPIs include:
- Turnaround Time (TAT): Measure the time taken from sample collection to result reporting.
- Error Reduction: Track the frequency of errors in data entry, reporting, or results.
- Operational Efficiency: Analyze workflow bottlenecks and compare pre- and post-implementation productivity.
- Staff Satisfaction: Use surveys to gauge user experience and ease of use.
Monitor Workflow Improvements
- Evaluate how the LDMS has streamlined processes such as:
- Sample tracking and test assignment.
- Integration with laboratory instruments.
- Report generation and distribution.
- Compare time and effort spent on tasks before and after implementation.
Assess Financial Impact
- Calculate the return on investment (ROI) by comparing costs and benefits:
- Cost Savings: Reduction in errors, manual tasks, and downtime.
- Revenue Growth: Faster processing and higher accuracy can improve client retention and attract new business.
- Factor in ongoing costs like system maintenance and updates.
Ensure Compliance Goals Are Met
- Verify that the system has helped your lab maintain or enhance compliance with industry regulations.
- Check that audit trails, reporting features, and data security measures are functioning as intended.
Track Staff Adaptation
- Measure how quickly staff have adopted the system and become proficient. Indicators include:
- Time taken to complete tasks with the LDMS.
- Feedback on the ease of learning and using the system.
- Identify staff who may need additional training or support.
Analyze Patient Outcomes
- Monitor whether the system has contributed to better patient care by:
- Reducing delays in diagnostic results.
- Improving accuracy in test reporting.
- Enhancing communication between labs and healthcare providers.
Review Scalability and Flexibility
- Assess whether the system can handle:
- Increased test volumes.
- Integration with new equipment or technologies.
- Adaptation to changes in workflows or regulations.
Collect Feedback from Stakeholders
- Gather input from all parties involved, including:
- Lab staff: Day-to-day users of the system.
- IT professionals: Responsible for system maintenance.
- Hospital administrators: Focused on ROI and strategic outcomes.
- Use this feedback to identify areas for further improvement.
Benchmark Against Industry Standards
- Compare your lab’s performance metrics with industry averages to gauge the system’s effectiveness.
- Look for best practices in similar labs that can inspire further optimization.
Document and Share Results
- Prepare a report summarizing the system’s impact. Include:
- Before-and-after comparisons of KPIs.
- Financial outcomes and ROI analysis.
- Success stories or notable improvements.
- Share this report with stakeholders to demonstrate value and build confidence in future projects.
Measuring success ensures you maximize the benefits of your LDMS and identify areas for continuous improvement. It’s a process that doesn’t stop after implementation but evolves alongside your lab’s needs.
Case Studies and Examples
Exploring real-world examples of successful Lab Diagnosis Management System (LDMS) implementations can provide valuable insights and inspire confidence in the process. These case studies highlight how various labs overcame challenges, optimized workflows, and achieved measurable outcomes.
Streamlining Workflows in a High-Volume Lab
- Background: A large hospital laboratory processing over 1,000 tests daily faced inefficiencies in sample tracking and result reporting.
- Challenges:
- Frequent delays in test processing.
- High error rates due to manual data entry.
- Difficulty maintaining compliance with CLIA standards.
- Solution: The lab implemented an LDMS with automated sample tracking and reporting features.
- Outcomes:
- Reduced turnaround time by 35%.
- Achieved near-zero errors in data entry.
- Improved compliance audit readiness through robust documentation tools.
- Takeaway: Automation and streamlined workflows enabled this lab to handle higher volumes with fewer errors.
Enhancing Data Security for a Private Diagnostic Center
- Background: A mid-sized private diagnostic center prioritized patient data privacy while expanding its operations.
- Challenges:
- Compliance with GDPR for handling sensitive patient information.
- Securing data across multiple locations.
- Solution: An LDMS with end-to-end encryption and role-based access controls was deployed.
- Outcomes:
- Improved data security through encryption and restricted access.
- Centralized data storage for better accessibility and monitoring.
- Passed multiple regulatory audits without concerns.
- Takeaway: Investing in data security features is essential for labs handling sensitive patient information.
Improving ROI for a Small-Scale Laboratory
- Background: A small-scale lab wanted to justify the cost of implementing an LDMS while improving operational efficiency.
- Challenges:
- Limited budget for system procurement and training.
- Resistance from staff accustomed to manual workflows.
- Solution: The lab opted for a modular LDMS that allowed gradual feature upgrades and implemented a phased training program for staff.
- Outcomes:
- ROI achieved within 18 months due to reduced operational costs and faster test processing.
- Increased staff satisfaction and productivity.
- Scaled operations by adding new modules as the lab grew.
- Takeaway: Modular systems and phased implementations help small labs maximize their investment.
Integration Success in a Multi-Site Hospital Network
- Background: A hospital network with multiple sites needed a centralized system to unify its diagnostic services.
- Challenges:
- Inconsistent workflows across sites.
- Difficulty sharing data between locations.
- Solution: An LDMS with cloud-based integration was implemented across all sites.
- Outcomes:
- Standardized workflows across the network.
- Real-time data sharing between locations, reducing duplication and delays.
- Better decision-making through centralized reporting and analytics.
- Takeaway: Cloud-based solutions enable seamless integration for multi-site operations.
Conclusion
Implementing a Lab Diagnosis Management System (LDMS) is a transformative step for any laboratory. By streamlining workflows, improving accuracy, and ensuring compliance, an LDMS empowers labs to meet growing demands while delivering better patient outcomes.
Throughout this guide, we’ve covered every stage of the process—from planning and system selection to implementation and post-launch support. Along the way, we’ve highlighted the importance of aligning the system with your lab’s unique needs and continuously measuring its success to drive improvements.